The Pharmaceutical Translation Process: Importance & Challenges

These translations must be correct, as mistakes can cost lives. Accurate translations protect patient safety by ensuring proper use and understanding of medicines, as well as enabling regulatory approval by meeting submission standards.
This article covers the core fundamentals of pharmaceutical translations, their importance, and the challenges associated with them – so you can protect the safety of patients, staff, and clinical trial participants.
What Are Pharmaceutical Translations?
Pharmaceutical translation is the specialized practice of converting drug and healthcare-related materials from one language into another while preserving exact meaning, clinical accuracy, legal intent, and adhering to local regulatory requirements.
These translations require translators with medical knowledge, familiarity with local regulations, and strict quality controls so that dose instructions, safety information, and trial details remain unambiguous and reliable.
Common Pharmaceutical Materials That Must Be Translated
Several common pharma materials require regular translation, including:
- Informed Consent Forms (ICFs): Documents that explain a trial or treatment, risks, and benefits to participants so they can give informed consent; clarity is legally and ethically critical.
- Patient Reported Outcomes (PROs): Questionnaires that capture patients’ own reports of symptoms or quality of life; translations must keep the questionnaire’s measurement properties intact.
- Electronic Patient Reported Outcomes (ePROs): The digital versions of PROs (apps, web forms) also require checks for UI strings and screen-layout constraints.
- Instructions for Use (IFUs): Step-by-step user guides for devices or medicines; wording must prevent misuse and ensure patient safety.
- Brochures: Patient-facing or HCP-facing marketing and informational sheets; need to be accurate and culturally appropriate.
- Training Documents: Materials for healthcare professionals or study sites (e.g., site manuals, SOPs); must convey procedures exactly to avoid errors.
- Synopses: Short summaries of study protocols or clinical data; they must faithfully reflect the original content because regulators and stakeholders often rely on them.
What Is the Pharmaceutical Translation Process?
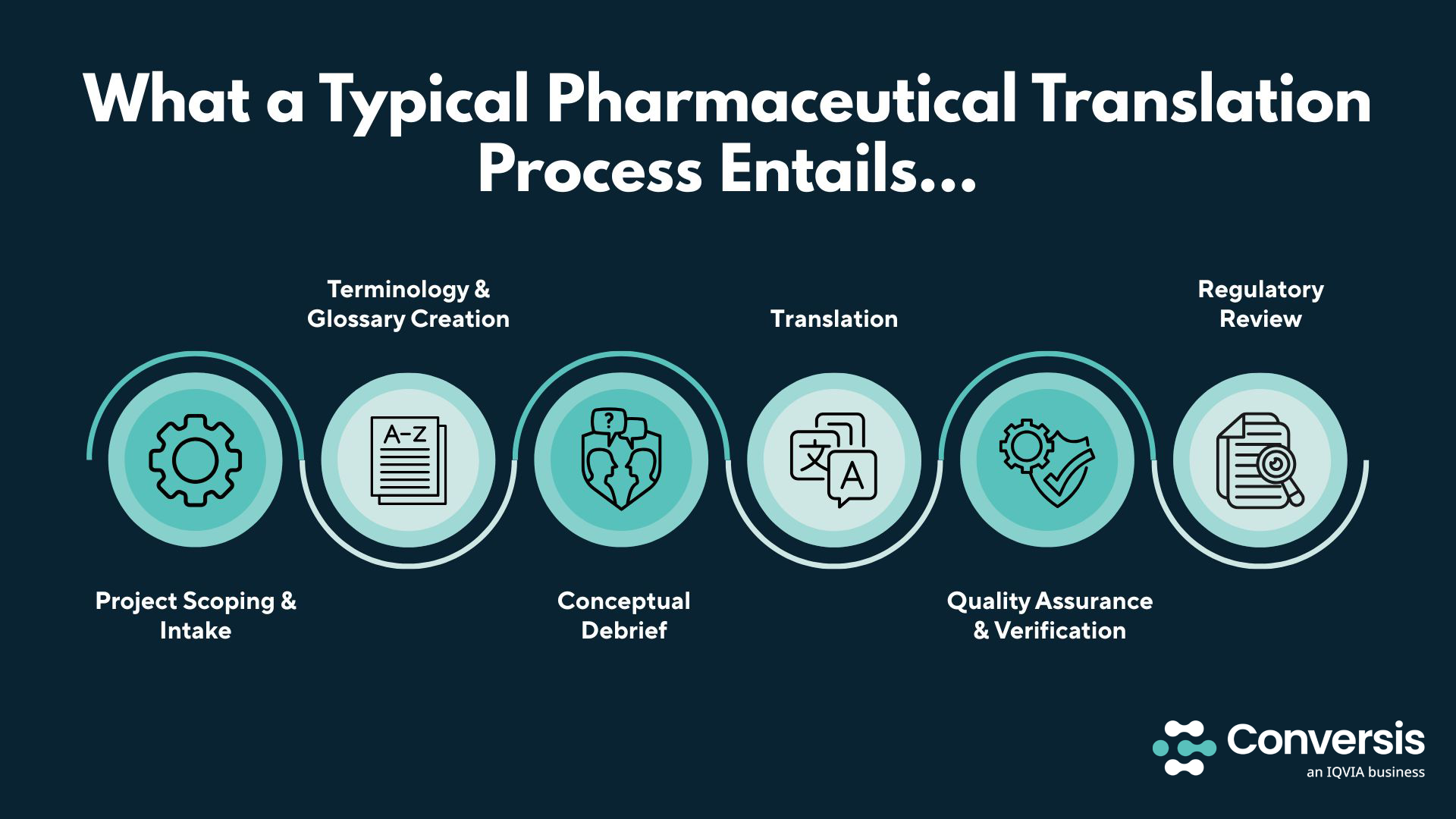
Pharmaceutical translations follow a highly-structured and quality-controlled process to ensure every word is accurate, consistent, and compliant with regulatory standards. However, every translation agency will have its own approach. This is why it’s important to choose a translation partner that is both open about how they approach the translation process and works in a way that is right for your organization.
Below, we have outlined a typical, industry-standard pharmaceutical translation process, with some additional information on where our approach may differ, based on years of experience delivering high-quality life science translation services.
1. Project Scoping & Intake
At the start of a project, the translation team reviews the source materials, target languages, and intended use of the documents. This stage defines timelines, delivery formats, and any national-specific requirements. It also ensures everyone understands the project’s scope before work begins.
2. Terminology & Glossary Creation
A dedicated glossary is developed to ensure consistent use of scientific and medical terms across all translations. This includes drug names, abbreviations, and technical vocabulary verified against official sources and client-preferred terminology. Having a glossary in place prevents ambiguity and maintains uniformity across every document.
3. Conceptual Debrief
Before translation begins, linguists and subject-matter experts discuss the intent and context of the materials. This step clarifies any potentially ambiguous sections and ensures that cultural nuances, patient comprehension levels, and regulatory tone are well understood. The goal is to align linguistic accuracy with clinical and regulatory intent.
4. Translation
As a general approach, professional translators – often with scientific or pharmaceutical backgrounds – translate using secure, compliant translation management systems. They follow the approved glossary and reference materials while maintaining the document’s technical integrity, readability, and local language conventions.
At Conversis, an IQVIA business, we only work with translators who possess the specific scientific, clinical, or pharmaceutical expertise required for each project – not generalists – ensuring subject-matter knowledge is applied in every translation.
5. Quality Assurance & Verification
Once the translation is complete, it undergoes multiple rounds of review. Proofreaders check for linguistic accuracy, formatting consistency, and adherence to regulatory and client standards.
6. Regulatory Review
Where applicable, the final stage involves validation by regulatory experts to ensure that the translated documents meet submission requirements in each target market. This review verifies compliance with authorities such as the EMA, MHRA, or FDA, confirming that every element – from terminology to layout – aligns with official standards before approval or public use.
What Are the Benefits of Pharmaceutical Translations?
Accurate pharmaceutical translations aren’t just a nice-to-have: they’re essential for pharmaceutical and biotechnology companies, clinical research organizations, and many others operating in global markets. Partnering with an accredited, certified translation agency helps organizations:
- Enhance Patient Safety & Care: Clear, accurate translations ensure patients understand dosages, warnings, and side effects, reducing medication errors and harm.
- Meet Regulatory Compliance: Localized, compliant documents meet health authority requirements, reducing the chance of rejected submissions or inspection issues.
- Accelerate Drug Development: Precise cross-border communication between sponsors, CROs, and regulators reduces rework and speeds up trials and approvals.
- Access Global Markets: Professionally-localized labels, information sheets, and marketing enable entry into new countries and reimbursement systems.
- Improve Healthcare Outcomes: When clinicians and patients correctly understand treatments, adherence and clinical effectiveness increase.
- Reduce Legal & Financial Risks: Accurate translations lower the risk of recalls, fines, litigation, and costly corrective actions due to misinterpretation.
- Build Trust & Reputation: Clear, culturally-sensitive communication builds credibility with patients, healthcare professionals, and regulators.
What Are the Main Challenges of Pharmaceutical Translations?
Ensuring accuracy and compliance in pharmaceutical translation is complex, and it must be performed correctly. Here are the main challenges that both healthcare organizations and translators face:
Complex Medical Terminology & Abbreviations
Pharmaceutical texts are filled with highly-technical language, clinical jargon, and abbreviations that can have different meanings depending on the context. Translators must have both medical expertise and linguistic precision to interpret these correctly without losing nuance or introducing errors.
Drug Names & Nomenclature
Drug names can vary between countries, and even minor differences in spelling or formatting can cause confusion or regulatory rejection. Translators must verify generic, brand, and chemical names against official databases such as the INN (International Nonproprietary Names) list to maintain accuracy and consistency.
Regulatory Variability
Each nation has its own approval requirements and documentation standards – for example, the EMA in Europe or the FDA in the United States. Keeping up with evolving regulations and ensuring compliance across all markets is one of the most demanding aspects of pharmaceutical translation.
Time Pressure & Version Control
Tight project timelines are common, especially when translation is needed for time-sensitive submissions or clinical trial milestones. Coordinating multiple translators across languages while maintaining version control and consistency can be challenging without robust translation management systems.
Confidentiality & Data Security
Pharmaceutical documents often contain sensitive patient data and unpublished research. Maintaining strict confidentiality, ensuring secure data handling, and complying with regulations such as GDPR and HIPAA are essential throughout the translation process to protect both patients and companies.
What Are the Best Practices for Pharmaceutical Translations?
Given the potential issues mentioned above, implementing several best practices is key to achieving the highest quality pharmaceutical translations. These include:
Using Specialist Language Service Providers (LSPs)
Work with LSPs (like Conversis) that specialize in life sciences – they provide translators with clinical and regulatory experience, validated workflows, and familiarity with national health authorities. A specialist partner also brings tested processes for audits, regulatory submissions, and post-market changes, reducing both risk and rework.
Build & Maintain Glossaries & Translation Memories
Create and continuously update centralized glossaries to maintain consistency in terminology, tone, and formatting across projects and markets. Use translation memories (TMs) to capture previously-approved segments so future work is faster, cheaper, and more consistent while preserving regulatory-approved phrasing.
Implement Multi-Stage Quality Assurance
Adopt a layered QA approach that includes checks such as linguistic review, editing, in-country review (when required), and, where appropriate, cognitive debriefing for patient-facing instruments to validate meaning and measurement equivalence.
Treat Labeling & Safety Text With Extra Controls
Apply stricter controls and bespoke workflows for labels, patient sheets, and adverse-event reporting text. Use mandatory sign-offs, automated checks for numerical and unit consistency, and parallel review by regulatory experts. These texts should not be treated like just any copy – they require the highest accuracy and traceability.
Add Robust Project Management, Version Control & Security
Use a secure Translation Management System (TMS) to manage versions, track changes, route approvals, and maintain audit trails; enforce role-based access, NDA procedures, and encrypted data transfers to comply with GDPR/HIPAA and protect proprietary data. Clear project governance reduces errors, prevents misaligned versions, and maintains regulatory readiness.
The Pharmaceutical Translation Process in Action
Conversis partnered with one of the world’s largest Contract Research Organizations (CROs) to localize patient-recruitment materials for three global clinical trials investigating a treatment for obesity and diabetes. The project covered around 140 language combinations across three concurrent trials, with large documents, tight deadlines, and frequent content updates.
Each item went through a six-stage workflow with differing approval schedules per country. We began translating early drafts to spread workload, using translation memories to minimize rework as content evolved. We applied strict version tracking, standardized file naming and formatting, and coordinated multiple project managers, QA, and DTP teams to handle last-minute changes smoothly.
The Outcome
All multilingual materials were delivered on time, enabling the client to meet submission deadlines and begin patient enrollment within two months. The result demonstrated how a disciplined, scalable translation process ensures regulatory-ready materials – protecting accuracy, timelines, and global trial integrity.
Get In Touch to Discuss Our Pharmaceutical Translation Services
Inaccurate or inconsistent translations can put patient safety at risk and expose your organization to serious legal and financial consequences. With Conversis, you can avoid these risks through expertly managed pharmaceutical translation services designed to meet the highest linguistic and regulatory standards.
Our team of life sciences specialists ensures that every document is handled with precision, confidentiality, and full compliance across markets. Contact us today to learn more about how we can help protect patients and support successful global market access.



